Tuesday, September 20, 2011
Pacific scouting expedition
Friday, September 2, 2011
Home!
Gareth here, now back on dry land and relaxing at home with my family after a busy couple of days of off-loading equipment and personnel. Everyone is happy to be back, but it was an incredibly successful cruise and we're all feeling very satisfied. I am immensely proud of our team and all that we accomplished, all done with camaraderie and a smile. The cruise of course also owes much of its success to the superb efforts and abilities of Captain Diego and his officers and crew. Ocean-going research is all about teamwork between the bridge, crew, and science party, and this cruise to me represented the finest kind of collaboration.
A few numbers: all told we spent a whopping $695,184 on ship-time alone -- these kinds of expeditions are certainly expensive propositions. We traveled over 4300 nautical miles, a very impressive distance, equivalent to about twice the width of the US -- and all done at an average of about 12 miles an hour! We went through over a hundred gallons of ethanol to preserve our net samples, and collected hundreds of gigabytes of data.
Now we enter the next phase of the project, where we churn through those data and samples to glean as much information as possible on pteropods and their environment. By next year we'll no doubt have fresh insight into this problem that will help guide our continued fieldwork -- next summer we'll be back on the water, this time in the Pacific, to repeat the exact same sampling in the very different pH conditions of the northeast Pacific. Stay tuned to find out what we learn!
A few numbers: all told we spent a whopping $695,184 on ship-time alone -- these kinds of expeditions are certainly expensive propositions. We traveled over 4300 nautical miles, a very impressive distance, equivalent to about twice the width of the US -- and all done at an average of about 12 miles an hour! We went through over a hundred gallons of ethanol to preserve our net samples, and collected hundreds of gigabytes of data.
 |
| Limacina retroversa, a species of pteropod that proved to be very abundant in the northern part of our study area. These beautiful shells are only about a millimeter long! [Photo: Nancy Copley] |
Now we enter the next phase of the project, where we churn through those data and samples to glean as much information as possible on pteropods and their environment. By next year we'll no doubt have fresh insight into this problem that will help guide our continued fieldwork -- next summer we'll be back on the water, this time in the Pacific, to repeat the exact same sampling in the very different pH conditions of the northeast Pacific. Stay tuned to find out what we learn!
Thursday, September 1, 2011
Fun Aboard the Oceanus
Sunrise, Sunset, and Sea Life

 Photos by: Katie Wurtzell
Photos by: Katie Wurtzell
One of the great things about being at sea is that you are always in a perfect position to watch the sunrise or sunset (that is if you are awake and it is not overcast outside)! When the conditions are right, every morning or evening we gather outside to watch the either the first or last rays of light reflect on the sea. Also, whenever possible, we help Tim scout for sea life. Over the past few days we have seen many Shearwaters, Petrels, Turns, dolphins, sea turtles and pilot whales.

 Photos by: Katie Wurtzell
Photos by: Katie WurtzellA Little Cup is a BIG Deal
Prior to arriving at our mid-way day/night station, we made time for arts and crafts: decorating Styrofoam cups. These cups were attached to the CTD rosette and lowered to a depth of 3000m. As pressure increases with depth, the air from the cups is squeezed out, and thus the cups shrunk. The pictures, cruise information, and other messages drawn on the cups were deformed but serve as a unique souvenir from the trip.
Photos by: Nancy Copley
The Library
The library is a common place to hangout when we are off watch. Here, someone can always be found reading, playing cards, chatting, or watching movies.
Photo by: Peter Wiebe
And We Eat…
So far we have consumed:
350 lbs meat
150 lbs flour
120 lbs potatoes
60 lbs butter
30 lbs gummy bears
810 eggs
288 english muffins
240 soda (cans)
150 heads of lettuce
24 pineapple, cantaloupe, and honey dew
Photo by: Mark Anderson
-Katie and Katherine
Wednesday, August 31, 2011
This Critter Can Swallow Backwards!
This is a copepod, Sapphirina, that came up in the net one night. It's about 2 mm in length. It seems to be able to 'swallow' both down and up - at the same time! It also has two pair of lenses in front of the colored eyes. Take a look for yourself.
Tuesday, August 30, 2011
Pteropod Videos
The common names for pteropods are Sea Butterflies, for the shelled ones, and Sea Angels, for the shell-less or 'naked' ones. After viewing these short videos you'll understand why:
A Look Into the Sea
With the Video Plankton Recorder (VPR), an underwater video camera-microscope, we are able to take a unique look at the animals in the ocean while they are actually living there. Typically, pictures of plankton are taken in the lab in a dish or aquarium. It's hard to say for sure what their natural behavior would be when they're continuously bumping into glass walls but they're no doubt a bit stressed. The VPR is gently lowered through the water column with a strobe flashing 15 times per second. This may not be too natural for them either but it's a very brief encounter before the instrument passes them by. Some of the images are remarkable, take a look for yourself.
Here are a few images of pteropods and other plankton from the VPR.
Here are a few images of pteropods and other plankton from the VPR.
 |
| A pteropod, Limacina retroversa has a typical snail shaped body but it's foot has evolved into a pair of wings it uses to swim in the water column. |
 |
| another Limacina pteropod. |
 |
| This is a cone-shaped pteropod, Creseis |
 |
| A comb jelly floated by. |
 |
| A darkly colored amphipod with a light tail (left) and large eye (right). |
 |
| This copepod appears blue. |
 |
| This ostracod is swimming vertically, not what I would expect. |
Sunday, August 28, 2011
Survey's End!
Gareth here. We have now finished all of our 31 planned stations, and even had time to add on a 32nd 'icing-on-the-cake' station where we sampled some cold waters coming down along the Flemish Cap from the Labrador Sea.
We're all feeling a great sense of accomplishment as we now steam home. Some of our instruments, and of course our bird observer Tim 'The Bird Machine' White, that sample while the vessel is underway are still running, but otherwise most of the science party is now working on processing data and samples, and taking apart the equipment for when we return to Woods Hole. We're far enough offshore though that it will take us a total of about 6 days to make it home!
We're all feeling a great sense of accomplishment as we now steam home. Some of our instruments, and of course our bird observer Tim 'The Bird Machine' White, that sample while the vessel is underway are still running, but otherwise most of the science party is now working on processing data and samples, and taking apart the equipment for when we return to Woods Hole. We're far enough offshore though that it will take us a total of about 6 days to make it home!
 |
| Nancy and Katie washing down the nets from the MOCNESS, ready to be stowed away until the next cruise. [Photo: G. Lawson] |
 |
| The MOCNESS nets hanging out to dry from the 01 deck rail. [Photo: P. Wiebe] |
 |
| Gareth and Alex disconnecting the data cable from the "portable" winch installed for this cruise to allow us to tow the HamarHead broadband echosounder. [Photo: P. Wiebe]. |
Friday, August 26, 2011
Panoramas
Gareth here. Robb Hagg is the marine technician with WHOI's Shipboard Scientific Services Group who is out here with us making sure all of the ship's scientific equipment works properly, ranging from the CTD to the depth sounders to the printers to the ship's network and connectivity to the internet. On top of all that he has also been experimenting with a panoramic camera system that lets him take photos that cover 360 degrees instantaneously (i.e., no stitching together of images is required after the fact). Below are stills from some QTVRs (Quick Time Virtual Reality) he's created -- clicking on the links will take you to webpages on the WHOI site where you can see the movie files, and you should be able to navigate within them to explore all directions in the image.
And here is a more traditional panoramic photograph showing another science party meeting. These have been a very useful thing, giving the day watch and the night watch a chance to exchange information, hatch plans, and discuss any issues that have come up.
Click here to see the Day Watch Biology Team processing the catch from the MOCNESS net system.
Click here to see the nightly watch change-over all-hands meeting of the science party where we discuss the plan for the next 24 hours and discuss any other important issues.
Click here to see the nightly watch change-over all-hands meeting of the science party where we discuss the plan for the next 24 hours and discuss any other important issues.
And here is a more traditional panoramic photograph showing another science party meeting. These have been a very useful thing, giving the day watch and the night watch a chance to exchange information, hatch plans, and discuss any issues that have come up.
 |
| Photo: Peter Wiebe |
You can see more panoramas from a previous Oceanus cruise at: Dan Smith and Larry Madin's Virtual Stowaway site.
Thursday, August 25, 2011
How do we measure ocean acidification?
Hi, this is Aleck Wang. I am a scientist (marine chemist) from the Department of Marine Chemistry and Geochemistry, Woods Hole Oceanographic Institution. I study the marine carbon dioxide system, also the called carbonate system or inorganic carbon system. My lab group is responsible for measuring all primary CO2 parameters during the cruise. These include seawater pH, partial pressure of carbon dioxide (pCO2), dissolved inorganic carbon (DIC), and total alkalinity (TA). I would like to talk about how we determine or measure ocean acidification. You already know from our previous blog posts what ocean acidification (OA) is. Briefly, as we (humans) release more CO2 to the atmosphere through burning of fossil fuels and other activities, more and more CO2 is dissolved in the ocean, which causes the ocean water to be more acidic (lower pH). The process is similar to when one makes a soda water: as we inject CO2 to water, it becomes more acidic (the resulting soda is acidic).
 |
| Fig. 1. Mean pCO2 and pH in the upper ocean of the Central Pacific Ocean near Hawaii between 1988-2007 (Dore J. E. et al. PNAS 2009, 106:12235-12240) |
Fig. 1 shows direct observational evidence that ocean acidification is happening. The data were collected at a fixed station from the central Pacific near Hawaii between 1988 and 2007. Newer data are also available. The top panel basically shows that surface water pCO2 increased at the same pace as the atmospheric CO2 increase (~ +1.9 ppm per year) during this period. In the meantime, surface ocean pH (middle panel) was decreasing at a rate of ~ 0.02 per decade. This increase in pCO2 and decrease in pH in the surface ocean have also been observed in other ocean basins and some continental shelf regions during the past two decades. To determine if the ocean is acidifying is in fact very demanding scientific work. It took more than two decades of efforts from many scientists around the globe to measure seawater pH, pCO2, DIC, and TA precise and accurate enough to undoubtedly discern the acidification signal, such as shown in Fig. 1. Part of this is because the rate of pH decrease and pCO2 increase in the ocean is a small number on an annual basis. Our measurements have to be good and long enough for us to say for sure that ocean acidification is happening.
 |
| Fig. 2. pH profiles at 5 stations in the North Atlantic Ocean during the cruise. |
Take pH measurements for example. Thanks to the analytical improvement in the 1990’s, we are able to measure seawater pH precisely to the 3rd - 4th decimal place (±0.0004 pH units) based on the colorimetric principle. This allows us to see the gradual pH decrease shown in Fig. 1. In addition, we can see many subtle features of pH from the surface to the deep ocean (Fig. 2). These will inform us what processes occur in the water column and how they may affect pH values. It takes a great deal of effort to make these measurements. Just to give you a sense; two groups of people have to work two shifts of 12 hours almost non-stop to finish all the pH measurements on board of R/V Oceanus during this current cruise.
 |
| Fig. 3. pCO2 measurements along the cruise track. Two red circles indicate we went through two boundaries (fronts) between different water bodies, where show large pCO2 changes. |
There have been significant improvements on the technologies of how we measure these carbonate parameters during the recent decade. We can now measure all four carbonate parameters near-continuously and autonomously with high precision and resolution. There are even in-situ sensors to measure these parameters. These improvements have reduced the measurement cost and increased efficiency. Fig. 3 shows the pCO2 data we just collected during our cruise in the N. Atlantic Ocean using a fully automated pCO2 instrument to make continuous underway measurements of surface water pCO2 along the cruise track. Because the measurement of the instrument is quick (~ 1 minute per measurement) and precise (±1 µatm), we captured two water fronts (boundaries) where surface pCO2 underwent large changes within short distances (time). The fronts in the ocean are often places where high biological production occurs. DIC is also a very useful and important measure of the carbonate system. It is the sum of all carbonate, bicarbonate ions and dissolved CO2 in seawater. As ocean is acidifying, and more atmospheric CO2 is dissolved into the ocean, DIC will increase as well. Fig. 4. shows a few DIC profiles from the cruise. They are almost mirror images of the pH profiles. Now we can measure DIC precisely with only 0.1% error.
 |
| Fig. 4. DIC profiles at 4 stations in the North Atlantic Ocean during the cruise. |
In summary, we now can measure ocean acidification precisely and accurately by measuring the key parameters related to OA, and inform the public and policy makers how fast ocean is acidifying. There should be little doubt that the ocean is acidifying. The question is how the acidification will affect all aspects, from chemistry to biology, of the ocean.
Wednesday, August 24, 2011
Diel Vertical Migration (DVM)
Every evening the ocean experiences a dramatic change. As the sun begins to set, many species of zooplankton and fish come to the surface waters to feed. It is said to be the largest migration on earth in biomass and number of animals participating. It is called diel vertical migration (DVM). The word diel comes from the Latin for "day." There is an upwards migration in the evening and a downwards migration in the morning.
There are a few hypotheses as to why this is done, such as predator avoidance. It may be a trade off between the safety of the dark deep sea and the bounty of food, such as phytoplankton, at the surface. Phytoplankton occurs only in water shallow enough to for the sun's rays to reach. Another hypothesis is that it is more bioenergetically efficient to perform DVM than to just stay consistently at the surface, because metabolic rates will be lower in the cooler deep waters.
DVM is also important to understand from a chemical stance. The critters migrate up to the surface waters and proceed to eat all night. Because they return to depth during day, they bring carbon and nutrients down with them, digesting the food, and excreting those nutrients into the deep waters. This migration occurs very quickly; planktonic organisms have been known to travel up and down in the water column at more than 200m per hour (Wiebe 1990). Our preliminary results from this cruise are similar. Swimming 200m/hour for a 1cm planktonic organism is equivalent to a 6ft human swimming 36km per hour, six times faster the Michael Phelps' world record time!
There are a few hypotheses as to why this is done, such as predator avoidance. It may be a trade off between the safety of the dark deep sea and the bounty of food, such as phytoplankton, at the surface. Phytoplankton occurs only in water shallow enough to for the sun's rays to reach. Another hypothesis is that it is more bioenergetically efficient to perform DVM than to just stay consistently at the surface, because metabolic rates will be lower in the cooler deep waters.
DVM is also important to understand from a chemical stance. The critters migrate up to the surface waters and proceed to eat all night. Because they return to depth during day, they bring carbon and nutrients down with them, digesting the food, and excreting those nutrients into the deep waters. This migration occurs very quickly; planktonic organisms have been known to travel up and down in the water column at more than 200m per hour (Wiebe 1990). Our preliminary results from this cruise are similar. Swimming 200m/hour for a 1cm planktonic organism is equivalent to a 6ft human swimming 36km per hour, six times faster the Michael Phelps' world record time!
 |
| Cuvierina columnela, an example of a pteropod we have been seeing that is known to vertically migrate. Photo: N. Copley. |
We are interested in studying the DVM of pteropods! One of the key questions we're trying to answer in this project is whether the DVM of pteropods is affected by ocean acidification. By measuring their DVM in different regions with different chemical conditions, we can determine whether the depth to which the pteropods migrate is shallower in regions where pH is lower in the deep waters where they would usually migrate to. This is why some of our scientific stations are 18 hours long. At these “day-night” stations, we can perform each type of deployment twice; once at night, and once during the day – and compare the results. We perform day and night MOCNESS tows, HammarHead deployments, VPR casts, and deep CTD casts (see post from August 12 for a more detailed explanation about this equipment).
As the MOCNESS tow can sample the water column at a series of discrete depth intervals, we are able to detect these vertical shifts by looking at the species composition of the samples. For example, if we were to find many organisms of the same species in the 200 – 400m sample during the daytime tow, and at night the same species was found more frequently in the shallower tows, it might be indicative that that species performs a diel vertical migration.
We can also study DVM with acoustics. Plotting the acoustic backscatter data for one day allows us to see both the daytime, and the nighttime migration. In the figure below, you can see this inside the black ovals. You can also see a narrow constant band at around 200m; not all species perform these migrations.
We can also study DVM with acoustics. Plotting the acoustic backscatter data for one day allows us to see both the daytime, and the nighttime migration. In the figure below, you can see this inside the black ovals. You can also see a narrow constant band at around 200m; not all species perform these migrations.

An echogram illustrating DVM. See previous post for tips on reading an echogram!
This phenomena is not unique to the north Atlantic. It has been observed in all regions of the ocean and even lakes!
Reference:
Wiebe, P.H., Copley, N.J, and Boyd, S.H. 1992. Coarse-scale horizontal patchiness and vertical migration in newly formed Gulf Stream warm-core ring 82-H. Deep-Sea Research 39, Suppl. 1: 247-278.
Monday, August 22, 2011
Echograms
I have been working on processing the acoustic data from the HTI hull mounted echosounding system. Essentially, the way echosounders works is to send a pulse of sound (aka a 'ping') down into the water and then listen for the echoes that scatter off of the animals and other objects (e.g., the bottom) in its path. Our system operates at four frequencies that we ping on in sequence, and we use the 'backscattered' echo data to tell us something about the abundances and kinds of animals that are here.
The data are most easily displayed in something called an Echogram. Echograms are colorful figures that display the acoustic backscatter data in a way that is easy to understand. They are a snapshot of the water underneath the ship over a specified time, typically a day. By looking at all four frequencies, we can get an idea of what the various patches seen might be, since different kinds of animals scatter sound differently as we change the frequency. It's been very interesting to be able to see the changes in backscatter as we travel through different water masses!
Click on the picture below to enlarge and learn more about how to read an echogram!

Sunday, August 21, 2011
Denizens of the Deep
Hello, this is Alex. I want to tell you about some of the amazing animals that we are finding below 600 meters. Using the MOCNESS we capture samples as deep as 1000 meters, from a habitat unfamiliar to most people.
 |
| A deep sea sample gets poured through a sieve from the cod end of the net 1 (800-1000 m) [photo: A Bergan] |
Light is too low here for us to percieve, and those animals that are visual must either create their own light (biolumnescence) or have specially adapted eyes.

Red is the first wavelength to disappear as we descend to depth, so this deep red shrimp would simply look black, and have a better chance of hiding from predators than if it lived at the surface. [photo: A Bergan].
Another fact of deep sea living is that food is patchy in time and space. But if you move around too much in search of food, you become more likely to be ambushed and eaten. The key is to keep your prey once you find it, which is acomplished by large teeth as seen in the fish below.
 |
| [photo: A Bergan] |
 |
| [photo: K. Wurtzell] |
Many deep sea species have slow growth rates, but long lives and end up becoming rather large. We found our largest pteropod between 800-1000 m; see the Clio polita from Gareth's post on August 18th. Here is a particularly large amphipod who is also found in that depth range.
 |
| This gammarid amphipod has reduced eyes. For it the cost of sight outweighs the benefit. [photo: K. Wurtzell] |
The deep sea is full of mystery and we are only scratching the surface. Often the open ocean is deeper than 4000 meters, and we are only sampling to a quarter of this depth. What lies beneath? If this excites your sense of exploration, then deep sea oceanography is for you!
 |
A squid found between 600-800 meters. We also see squid from the deck at night. Does this species have a daily vertical migration? Is it a different species that stays at depth? Is this a juvenile that stays deep to grow up before rising to the surface? [photo: A Bergan] |
Saturday, August 20, 2011
Tour of the Engine Room
A few days ago, Alex and I took a tour of the engine room with Chief Engineer Gary! It was fascinating to see the inner workings of the ship – from what keeps the lights on, to what powers the ship – even how we create a constant supply of freshwater!
Before we entered the engine room, we needed to put on ear protection because it is very loud in there. The first thing we saw as we began our tour were the two 300KW diesel generators that are the source of all of the shipboard electricity.

Katie giving a "thumbs up!" next to one of the generators
The next thing we saw was the main engine – 16 cylinder, 1500 horsepower. The engine turns the propeller, which powers the ship through the water. The propeller is a controllable pitch propeller, which means the ships speed is controlled by both the rate of the propeller (RPM) and the pitch, or angle. Our typical cruising speed is about 10 knots. The ship also utilizes a bow thruster. The bow thruster helps with maneuvering. Both the engine and the generators are fueled by diesel; the ship typically burns between 1000 to 2000 gal a day and can hold up to 48,000 gallons.

Chief Engineer Gary next to the main engine. The white padding (lagging) is surrounding the exhaust pipes to keep the heat in.
As you might imagine, the engine gets very warm from running 24/7. Water is pumped around the engine to cool it (jacket water). Heat from the main engine is used to aid in the evaporation of seawater in the evaporator, which generates freshwater for drinking, bathing, and scientific purposes. The seawater is under vacuum, and can therefore boil at 160 degrees. The ship has the capacity to generate around 3,600 gallons of water a day.
Friday, August 19, 2011
A little about chemistry on the RV Oceanus
Hello, I am Jacinta Edebeli. I am a summer student fellow on board the RV Oceanus on this ocean acidification- pteropod cruise. I am on the Chemistry half of the team. I am going to give you some insight into what the chemistry team is doing - what we sample for and how we measure them. When we send the CTD rosette down to 1000m or 3000m, we most often collect water samples. We commonly collect samples from 16 Niskin bottles for the 1000m cast and 24 Niskin bottles from the 3000m depth. We collect these samples to measure inorganic carbon (carbon dioxide (CO2)) parameters, nutrients and salinity. The CO2 parameters give us information about ocean acidification.
Three of these parameters include alkalinity, dissolved inorganic carbon (DIC) and pH. We measure these three in the lab on the boat using separate measuring instruments (please refer to pictures). We also have the MICA (Multi-Parameter Inorganic Carbon Analyzer), an underway seawater inorganic carbon measuring instrument which measures sea surface water DIC, pH and the fourth sea water inorganic carbon parameter, CO2 fugacity (partial pressure of CO2 in sea water, also known as fCO2). The MICA also measures atmospheric CO2 (or pCO2). We also measure salinity on the boat. The nutrient samples are frozen till we get to land.
Katherine Hoering drawing water from the CTD for analysis
Three of these parameters include alkalinity, dissolved inorganic carbon (DIC) and pH. We measure these three in the lab on the boat using separate measuring instruments (please refer to pictures). We also have the MICA (Multi-Parameter Inorganic Carbon Analyzer), an underway seawater inorganic carbon measuring instrument which measures sea surface water DIC, pH and the fourth sea water inorganic carbon parameter, CO2 fugacity (partial pressure of CO2 in sea water, also known as fCO2). The MICA also measures atmospheric CO2 (or pCO2). We also measure salinity on the boat. The nutrient samples are frozen till we get to land.
| Mohammad Uddin measuring salinity |
 |
| Cris Luttazi setting up the DIC analyzer |
| Jacinta Edebeli working with the alkalinity titrator |
The presence of hydrogen ions implies some level of acidity, hence, we also measure pH. The higher the concentration of hydrogen ions, the lower the pH, and vice versa. Comparing these present measurements with previous ones, we can tell whether or not there are changes occurring that we can relate to physiological and geographical patterns observed in the pteropods.
Thursday, August 18, 2011
Pteropods and the Arts
Gareth here. We scientists are not the only ones inspired to work on pteropods and their response to ocean acidification. We have counterparts in the Arts who also find these beautiful little animals fascinating, and who express their concerns about the impact of ocean acidification on pteropods and marine ecosystems through their respective media.
Below, for instance, is a youtube version of Sam Lardner's song "Pteropods," which has become the unofficial anthem of our cruise:
Our group has also recently started a collaboration with Cornelia Kavanagh, a sculptor who often takes inspiration from nature and who is currently working on a series of pieces on pteropods, interpreting via the medium of sculpture the impacts of ocean acidification on these animals. Cornelia will be showing these works in her gallery in spring of 2012, and the plan is for us to provide some materials providing a scientific background on pteropods and their place in the food web. You can see some of Cornelia's wonderful earlier works at www.corneliakavanagh.com.
Below are some recent photos of various pteropod species we've caught in our net system over the past few days. Hopefully the pictures capture how delicate and beautiful these animals are, and you can appreciate why people are inspired to sing about/sculpt/study them!
Below, for instance, is a youtube version of Sam Lardner's song "Pteropods," which has become the unofficial anthem of our cruise:
Our group has also recently started a collaboration with Cornelia Kavanagh, a sculptor who often takes inspiration from nature and who is currently working on a series of pieces on pteropods, interpreting via the medium of sculpture the impacts of ocean acidification on these animals. Cornelia will be showing these works in her gallery in spring of 2012, and the plan is for us to provide some materials providing a scientific background on pteropods and their place in the food web. You can see some of Cornelia's wonderful earlier works at www.corneliakavanagh.com.
Below are some recent photos of various pteropod species we've caught in our net system over the past few days. Hopefully the pictures capture how delicate and beautiful these animals are, and you can appreciate why people are inspired to sing about/sculpt/study them!
 |
| Pteropods come in a variety of shell types and sizes. These Calvolinia inflexa are about 5mm in length and have a complex shape with delicate spines. [Photo: Nancy Copley] |
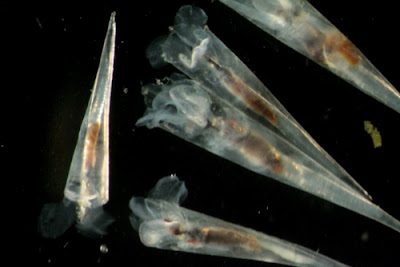 |
| These Styliola subula are about 4mm in length and have an elongated shell. [Photo: Nancy Copley] |
 |
| Clio polita, a rare species of pteropod and the largest we've caught so far. This individual came up in a net that sampled between 800 and 1000m below the surface. [Photo: Alex Bergan] |
Wednesday, August 17, 2011
Ocean Acidification: A Brief Synopsis
Hi, this is Cris Luttazi a WHOI Guest Student working in the Marine Chemistry and Geochemistry department. I thought a quick review of ocean acidification might be helpful.
Does the term ‘ocean acidification’ mean that seawater is becoming acidic?
 Carbon occurs naturally in seawater and without it marine life would cease to exist. There are many sources of dissolved carbon dioxide in oceanic waters including river input, hydrothermal vents and underwater volcanoes. Two significant sources are from the atmosphere and organic matter. Carbon dioxide is absorbed from the atmosphere as the Earth's oceans act as a carbon sink absorbing 1/3 of all anthropogenic carbon that comes into contact with the ocean's surface. Atmospheric gases are constantly being absorbed, dissolved and released from the ocean. The oceans hold roughly 50 times greater concentration of carbon dioxide per unit volume than the atmosphere. Another source of carbon dioxide is organic matter, derived from dissolved organic carbon decomposition, as food is broken down carbon dioxide is released.
Carbon occurs naturally in seawater and without it marine life would cease to exist. There are many sources of dissolved carbon dioxide in oceanic waters including river input, hydrothermal vents and underwater volcanoes. Two significant sources are from the atmosphere and organic matter. Carbon dioxide is absorbed from the atmosphere as the Earth's oceans act as a carbon sink absorbing 1/3 of all anthropogenic carbon that comes into contact with the ocean's surface. Atmospheric gases are constantly being absorbed, dissolved and released from the ocean. The oceans hold roughly 50 times greater concentration of carbon dioxide per unit volume than the atmosphere. Another source of carbon dioxide is organic matter, derived from dissolved organic carbon decomposition, as food is broken down carbon dioxide is released.

Too Much of a Good Thing
 Fig. 3. The Atlantic Ocean CCD Profile (Y-axis is depth in meters; the carbonate compensation depth is indicated by 1 and is shown in white. Below this depth the waters are corrosive to the form of calcium carbonate formed by pteropods.) (Diagram courtesy of Z. Wang).
Fig. 3. The Atlantic Ocean CCD Profile (Y-axis is depth in meters; the carbonate compensation depth is indicated by 1 and is shown in white. Below this depth the waters are corrosive to the form of calcium carbonate formed by pteropods.) (Diagram courtesy of Z. Wang).  Fig. 4. The Pacific Ocean CCD Profile (Y-axis is depth in meters) (Diagram courtesy of Z. Wang).
Fig. 4. The Pacific Ocean CCD Profile (Y-axis is depth in meters) (Diagram courtesy of Z. Wang).
Does the term ‘ocean acidification’ mean that seawater is becoming acidic?
In 2003, Ken Calderia coined the term ocean acidification in hopes of drawing attention to a critical marine issue. As many focused on the impacts of increased anthropogenic (human influenced or caused) carbon dioxide in the atmosphere, Ken was looking at the impact to our oceans. Less than a decade later, ocean acidification is the term used to describe the impact of increased carbon dioxide levels in the oceans. This topic has risen to the forefront of marine research, with a multitude of scientists, including our science party here on the Oceanus, trying to ascertain how marine organisms will acclimate to this environmental shift. This subject matter is important as each organism's ability to adapt will influence the food web and corresponding ecosystems.
SO, is the ocean becoming acidic?
Oceanic waters are naturally alkaline (pH scale 7.2 - 8.6 with a mean surface ocean pH of approximately 8.1). In comparison, lemon juice has a pH of 2.0 and bleach pH is nearly 13 (Fig. 5). However the ocean pH level is dropping or becoming less alkaline. All living organisms have a pH tolerance range, which is the organism's acidity tolerance. If the pH level drops or rises beyond the organism's tolerance, then it will cease to inhabit that location. Small changes in the water's pH can have enormous effects on species that have a limited pH tolerance range.
 Carbon occurs naturally in seawater and without it marine life would cease to exist. There are many sources of dissolved carbon dioxide in oceanic waters including river input, hydrothermal vents and underwater volcanoes. Two significant sources are from the atmosphere and organic matter. Carbon dioxide is absorbed from the atmosphere as the Earth's oceans act as a carbon sink absorbing 1/3 of all anthropogenic carbon that comes into contact with the ocean's surface. Atmospheric gases are constantly being absorbed, dissolved and released from the ocean. The oceans hold roughly 50 times greater concentration of carbon dioxide per unit volume than the atmosphere. Another source of carbon dioxide is organic matter, derived from dissolved organic carbon decomposition, as food is broken down carbon dioxide is released.
Carbon occurs naturally in seawater and without it marine life would cease to exist. There are many sources of dissolved carbon dioxide in oceanic waters including river input, hydrothermal vents and underwater volcanoes. Two significant sources are from the atmosphere and organic matter. Carbon dioxide is absorbed from the atmosphere as the Earth's oceans act as a carbon sink absorbing 1/3 of all anthropogenic carbon that comes into contact with the ocean's surface. Atmospheric gases are constantly being absorbed, dissolved and released from the ocean. The oceans hold roughly 50 times greater concentration of carbon dioxide per unit volume than the atmosphere. Another source of carbon dioxide is organic matter, derived from dissolved organic carbon decomposition, as food is broken down carbon dioxide is released. 
Fig. 1. The Carbon Cycle depicts how carbon moves among the atmosphere, the land and the oceans (Diagram courtesy of the United States Department of Energy 2011).
Too Much of a Good Thing
Since the industrial revolution, there has been a dramatic increase in atmospheric carbon dioxide (Fig. 2). The Intergovernmental Panel for Climate Change (IPCC) reported the average concentration of atmospheric carbon dioxide, as of July 2011, was 392.39 parts per million (ppm), which is 40% higher than pre-industrial levels of 280 ppm only 200 years ago (IPCC 2011). In contrast, carbon dioxide levels prior to the industrial revolution appear to have been fairly constant for at least the past thousand years.
In a natural system, without excessive anthropogenic carbon dioxide being released into the atmosphere, carbon dioxide values for the ocean and atmosphere are in equilibrium as the oceans buffer small fluctuations. But as atmospheric carbon dioxide increases, the equilibrium is disturbed as the ocean’s natural buffering system cannot accommodate the higher carbon dioxide input.
Fig. 2. Historical increase in atmospheric carbon dioxide concentrations (Diagram courtesy of the White House Inititative on Global Climate Change 2011) .
So why do we care? When carbon dioxide dissolves in seawater, carbonic acid is produced. However, carbonic acid is not stable in seawater and immediately separates or disassociates. This causes a release of hydrogen ions. This increase in hydrogen ion concentration increases the acidity of the seawater. When the hydrogen ions are released, they are able to combine with carbonate ions to form a bicarbonate ion thus reducing the amount of carbonate ions.
Calcifying marine organisms, such as corals and mussels, as well as the pteropods we are out here studying, exploit carbonate ions from calcium carbonate. When there is a decline in carbonate ions, the calcification rates decline proportionally. This means species that rely on carbonate ions to make shells are impacted.
Location, Location, Location
Carbon dioxide is not static in the water column. The movement of carbon dioxide is governed by two properties: the thermohaline circulation and carbon dioxide solubility properties in water. The thermohaline circulation pattern mixes surface waters with deeper waters through vertical and horizontal ocean currents driven by differences in water temperature and salinity levels. Colder, more saline water at higher latitudes is denser and sinks. As water temperature decreases, the solubility of carbon dioxide increases. Therefore, each cubic meter of cold water at higher latitudes carries more carbon dioxide than the same cubic meter of warmer water at the equator. The point being that the chemistry of the oceans varies with depth and latitude.
The result is that there is a depth in the ocean, the carbonate compensation depth (CCD), below which level calcium carbonate (shells) start to dissolve. The depth of the CCD is dependent on water temperature, pressure and salinity and varies within and also between oceans. The two diagrams below depict the different profiles of the Atlantic and Pacific Oceans (Fig. 3 and Fig. 4) along the study transect we are presently surveying (in the Atlantic) and along the transect we plan to survey next summer (in the Pacific). As you can see from these figures, in the modern-day North Atlantic the CCD is deeper than 2000m right up to 50 degrees North. By 2100, however, under ocean acidification the CCD is predicted to shoal to as shallow as 100m at northern latitudes. In contrast, in the modern-day Pacific, the CCD is already naturally much shallower and varies with latitude, from about 700m at 35 degrees North to about 150m at 50 degrees North. Our goal is to capitalize on these differences between and within ocean basins to understand how seawater chemistry influences the abundance, vertical range, and types of pteropods found in the water column.
 Fig. 3. The Atlantic Ocean CCD Profile (Y-axis is depth in meters; the carbonate compensation depth is indicated by 1 and is shown in white. Below this depth the waters are corrosive to the form of calcium carbonate formed by pteropods.) (Diagram courtesy of Z. Wang).
Fig. 3. The Atlantic Ocean CCD Profile (Y-axis is depth in meters; the carbonate compensation depth is indicated by 1 and is shown in white. Below this depth the waters are corrosive to the form of calcium carbonate formed by pteropods.) (Diagram courtesy of Z. Wang).  Fig. 4. The Pacific Ocean CCD Profile (Y-axis is depth in meters) (Diagram courtesy of Z. Wang).
Fig. 4. The Pacific Ocean CCD Profile (Y-axis is depth in meters) (Diagram courtesy of Z. Wang). On our current R/V Oceanus cruise we are using the variety of instruments described in an earlier blog post to determine the abundance and diversity, as well as the vertical and horizontal distribution, of pteropods, while concurrently analyzing the ocean's carbonate chemistry and specifically the calcium carbonate saturation levels along a transect from 35 to 50 degrees North in the Atlantic. Next year we'll make the same measurements along the same latitudinal range in the Pacific. This research will give us baseline information on pteropod ecology, and will hopefully give us an improved understanding of how pteropods are likely to react to the continued changing chemistry of the oceans.
Subscribe to:
Posts (Atom)













